Researchers redefine gene involved with DNA repair
Study on yeast cells reveals how gene affects speed of damage caused by UV radiation
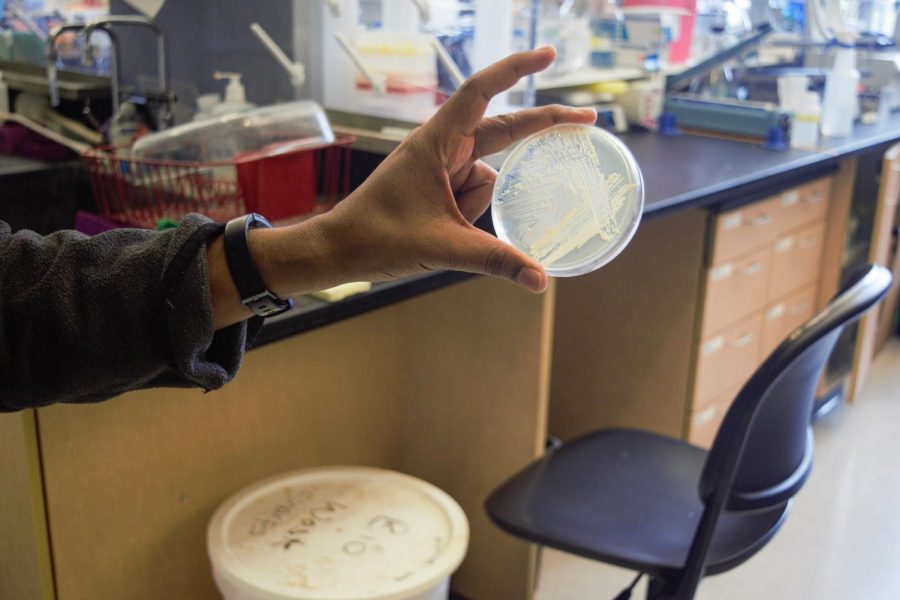
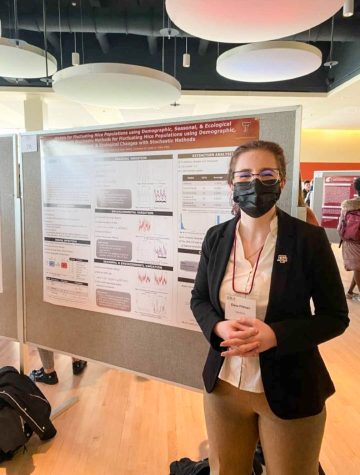
Postdoctoral researcher Kathir Selvam holds a sample of yeast cells after the Elf1 gene has been knocked out.
September 2, 2021
WSU researchers discovered a gene involved in repairing genetic pathways that can lead to cancerous conditions, neurological defects and cause aging.
The gene — named ELOF1 — was discovered 20 years ago in humans. It was solely recognized for its role in transcribing DNA. The WSU research team is the first to recognize its role in repairing damaged DNA from UV radUnaiation, said Kathir Selvam, postdoctoral researcher with the WSU School of Molecular Biosciences.
Selvam said he and his team cannot test ELOF1 because it is only found in humans, so they are studying its genetic counterpart, Elf1, which can be found in yeast cells. By exposing the cells to UV light, which can cause cancerous conditions or premature aging, the researchers are testing Elf1’s role in DNA repair.
In the yeast cell trials, Elf1 is “knocked out” of the yeast. The cells, which no longer have Elf1, are exposed to UV light in a controlled environment for about one week. When Elf1 is reintroduced to the cell, it is repaired in about one day.
To analyze the process, John Wyrick, WSU School of Molecular Biosciences associate professor, said a genetic sequencing method was developed. The sequence maps where the damage, which can be randomly distributed, forms across a genome.
Genome maps are then compared to analyze the repairing properties of the Elf1 gene, Selvam said.
So far, results show that cells die more readily when Elf1 is removed following UV irradiation, Wyrick said.
Because Elf1 and ELOF1 are counterparts, Elf1’s effect in yeast cell’s DNA repair pathway would be similar to ELOF1’s effect in human DNA repair, Selvam said.
Wyrick said he and Selvam’s group are collaborating with researchers at other universities. This includes the Erasmus University Medical Center in the Netherlands, which focuses on ELOF1’s effect on human cells.
Wyrick said the research began in summer 2019 and is centered around studying how DNA is damaged, resulting in possible mutations in genes. The research’s goal was to study a specific type of DNA damage resulting from exposure to UV light.
Nucleotide excision repair is a critical repair pathway that helps repair cell damage, Wyrick said. When there are genetic defects in a DNA pathway, it significantly increases the likelihood an individual will have cancer.
NER repairs damage to DNA lesions caused by UV exposure, Wyrick said. Normally, NER goes along the pathway, searching for distorted DNA lesions and tries to repair them.
“It’s like finding a needle in a haystack,” he said.
Typically, DNA is transcribed into RNA by a protein called RNA polymerase. But when the DNA is damaged, the RNA polymerase stalls and the damage has to be repaired.
Failure in this repair can cause a number of human diseases, including Cockayne syndrome — a rapidly aging disease that can cause neurological defects and prevent individuals from surviving past their teen years — and make an individual more susceptible to cancerous conditions, Selvam said.
“Essentially it’s like a car going along and if a cow gets in your way, then you stop the car,” Wyrick said.
If something goes wrong when DNA is transcribed into RNA, a NER subpathway is initiated to repair the damage so transcription can continue.
“The cow then gets moved out of the way and the car keeps driving,” Wyrick said.
Wyrick said ELOF1 likely plays a role in the pathway because it binds to the RNA polymerase and travels with it, helping to repair and bypass the damage.
“Basically, we want to understand what genes are playing a role in that [repair] pathway, and what role they play,” Wyrick said.
ELOF1 is not exclusive to repairing damage from UV exposure. It is equally essential in repair pathways found in human genetic diseases or mutations, Wyrick said.
In previous research, Wyrick said mouse embryos were not able to develop without the presence of the ELOF1 gene, indicating it is likely needed for survival.
“The research is just focusing on understanding the mechanisms, understanding how the damages are bad,” Selvam said. “And long term, understanding how mechanisms can, of course, lead to improving … treatment.”